Wednesday, December 29, 2021
Friedreich's Ataxia Frequency in a Large Cohort of Genetically Undetermined Ataxia Patients
Sunday, December 26, 2021
The power and the promise of CRISPR/Cas9 genome editing for clinical application with gene therapy
The classification of neurodegenerative disease from acoustic speech data
Objective Assessment of Progression and Disease Characterization of Friedreich Ataxia via an Instrumented Drinking Cup: Preliminary Results
Conformational stability, dynamics and function of human frataxin: Tryptophan side chain interplay
Thursday, December 16, 2021
Characterizing cardiac phenotype in Friedreich's ataxia: The CARFA study
Wednesday, December 15, 2021
Myocardial and Arrhythmic Spectrum of Neuromuscular Disorders in Children
Tuesday, December 14, 2021
Friedreich cardiomyopathy is a secondary desminopathy
Saturday, December 11, 2021
Epigenetic Heterogeneity in Friedreich Ataxia Underlies Variable FXN Reactivation
Indefinite suspension of further development of the Company’s XCUR-FXN program for the treatment of Friedreich’s ataxia
The results of the investigation are summarized below.
Beginning in the autumn of 2020, Dr. Corbett misreported raw data from certain research and development experiments related to XCUR-FXN;
Dr. Corbett misreported the results of at least three different experiments that were conducted through at least February 2021;
The misreported data related solely to efficacy rather than safety of XCUR-FXN;
The misreported data was included in various public presentations and SEC filings from as early as January 7, 2021 through as late as August 12, 2021;
Indefinite suspension of further development of the Company’s XCUR-FXN program for the treatment of Friedreich’s ataxia
Douglas Feltner, M.D., the Company’s Chief Medical Officer, has agreed to assist in the wind down of the cavrotolimod and XCUR-FXN programs and will depart the Company on January 31, 2022.
Thursday, December 9, 2021
Rad9-mediated checkpoint activation is responsible for elevated expansions of GAA repeats in CST-deficient yeast
Longitudinal Assessment Using Optical Coherence Tomography in Patients with Friedreich’s Ataxia
Friday, December 3, 2021
The Responsiveness of Gait and Balance Outcomes to Disease Progression in Friedreich Ataxia
Wednesday, December 1, 2021
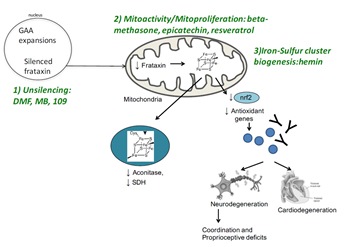
Nuclear Factor Erythroid-Related Factor 2 Signaling in the Neuropathophysiology of Inherited Metabolic Disorders
Monday, November 29, 2021
Source of medical information and behavioral seeking patterns in patients affected with Friedreich's ataxia and their caregivers: a survey study
Tuesday, November 23, 2021
Diagnosis and Management of Cardiovascular Involvement in Friedreich Ataxia
Monday, November 22, 2021
Cellular pathophysiology of Friedreich's ataxia cardiomyopathy
Friday, November 19, 2021
Exicure, Inc. Reports Third Quarter 2021 Financial Results and Corporate Progress
Reata Pharmaceuticals Receives Fast Track Designation From the FDA for Omaveloxolone for the Treatment of Friedreich’s Ataxia
Thursday, November 18, 2021
Body Mass Index and Height in Friedreich Ataxia What Do We Know?
Tuesday, November 16, 2021
STEALTH BIOTHERAPEUTICS REPORTS THIRD QUARTER 2021 FINANCIAL RESULTS AND RECENT BUSINESS HIGHLIGHTS
Larimar expects to initiate its Jive open-label extension and pediatric multiple ascending dose trials in the first half of next year.
Sunday, November 14, 2021
Body Mass Index and Height in the Friedreich Ataxia Clinical Outcome Measures Study
Saturday, November 13, 2021
Friedreich's ataxia. The investigator is awaiting Institutional Review Board approval of the planned Phase 2a clinical trial in patients with Friedreich's ataxia and expects to begin enrollment in early 2022.
Wednesday, November 10, 2021
Clinical Initiation of Lead GeneTACTM Program for Friedreich Ataxia On-track for the First Half of 2022
Tuesday, November 9, 2021
Molecular approaches for the treatment and prevention of Friedreich's ataxia
Sunday, November 7, 2021
Designing phase II clinical trials in Friedreich ataxia
Friday, November 5, 2021
Construction of DNA/RNA Triplex Helices Based on GAA/TTC Trinucleotide Repeats
Thursday, November 4, 2021
Longitudinal investigation of brain activation during motor tasks in Friedreich ataxia: 24-month data from IMAGE-FRDA
Tuesday, November 2, 2021
Clinical and Molecular Features of First Mexican Friedreich's Ataxia Patients with Compound Heterozygous FXN Mutations
Saturday, October 30, 2021
Left atrial appendage thrombosis in a patient with Friedreich Ataxia–related cardiomyopathy, left ventricular systolic dysfunction, and atrial fibrillation
Tract-Specific Spinal Cord Diffusion Tensor Imaging in Friedreich's Ataxia
Friday, October 29, 2021
Targeting 3′ and 5′ untranslated regions with antisense oligonucleotides to stabilize frataxin mRNA and increase protein expression
Yanjie Li, Jixue Li, Jun Wang, David R Lynch, Xiulong Shen, David R. Corey, Darshan Parekh, Balkrishen Bhat, Caroline Woo, Jonathan J Cherry, Jill S Napierala, Marek Napierala, Nucleic Acids Research, 2021;, gkab954, doi:10.1093/nar/gkab954
Friedreich’s ataxia (FRDA) is a severe multisystem disease caused by transcriptional repression induced by expanded GAA repeats located in intron 1 of the Frataxin (FXN) gene encoding frataxin. FRDA results from decreased levels of frataxin; thus, stabilization of the FXN mRNA already present in patient cells represents an attractive and unexplored therapeutic avenue. In this work, we pursued a novel approach based on oligonucleotide-mediated targeting of FXN mRNA ends to extend its half-life and availability as a template for translation. We demonstrated that oligonucleotides designed to bind to FXN 5′ or 3′ noncoding regions can increase FXN mRNA and protein levels. Simultaneous delivery of oligonucleotides targeting both ends increases efficacy of the treatment. The approach was confirmed in several FRDA fibroblast and induced pluripotent stem cell-derived neuronal progenitor lines. RNA sequencing and single-cell expression analyses confirmed oligonucleotide-mediated FXN mRNA upregulation. Mechanistically, a significant elongation of the FXN mRNA half-life without any changes in chromatin status at the FXN gene was observed upon treatment with end-targeting oligonucleotides, indicating that transcript stabilization is responsible for frataxin upregulation. These results identify a novel approach toward upregulation of steady-state mRNA levels via oligonucleotide-mediated end targeting that may be of significance to any condition resulting from transcription downregulation.