Rosa, P.L.; Bertini, E.S.; Piemonte, F.; Int. J. Mol. Sci. 2020, 21, 916. doi:10.3390/ijms21030916
Frataxin depletion impairs iron–sulfur cluster biosynthesis and determines iron accumulation in the mitochondria. Mounting evidence suggests that these defects increase oxidative stress susceptibility and reactive oxygen species production in FA, where the pathologic picture is worsened by a defective regulation of the expression and signaling pathway modulation of the transcription factor NF-E2 p45-related factor 2 (NRF2), one of the fundamental mediators of the cellular antioxidant response. NRF2 protein downregulation and impairment of its nuclear translocation can compromise the adequate cellular response to the frataxin depletion-dependent redox imbalance. As NRF2 stability, expression, and activation can be modulated by diverse natural and synthetic compounds, efforts have been made in recent years to understand if regulating NRF2 signaling might ameliorate the pathologic defects in FA. Here we provide an analysis of the pharmaceutical interventions aimed at restoring the NRF2 signaling network in FA, elucidating specific biomarkers useful for monitoring therapeutic effectiveness, and developing new therapeutic tools.
Friday, January 31, 2020
Tuesday, January 28, 2020
Europe’s drug regulator wins court backing for data transparency policy
Clare Dyer, BMJ 2020;368:m302
The European Union’s highest court has decisively upheld the European Medicines Agency’s right to publish clinical trial data from drug companies’ applications to market their products in the EU.
In two keenly awaited judgments, the European Court of Justice has comprehensively rejected appeals by two companies against rulings in 2018 by the General Court of the European Union upholding the European Medicines Agency’s open data policy.12
In 2010 the agency adopted a policy of making available all documents underlying a successful drug licence application from January 2014, setting aside its previous presumption of confidentiality. But the move was resisted by pharmaceutical companies, which mounted legal challenges, arguing that their commercial confidentiality was at risk.
The European Union’s highest court has decisively upheld the European Medicines Agency’s right to publish clinical trial data from drug companies’ applications to market their products in the EU.
In two keenly awaited judgments, the European Court of Justice has comprehensively rejected appeals by two companies against rulings in 2018 by the General Court of the European Union upholding the European Medicines Agency’s open data policy.12
In 2010 the agency adopted a policy of making available all documents underlying a successful drug licence application from January 2014, setting aside its previous presumption of confidentiality. But the move was resisted by pharmaceutical companies, which mounted legal challenges, arguing that their commercial confidentiality was at risk.
Monday, January 27, 2020
Availability, accessibility and delivery to patients of the 28 orphan medicines approved by the European Medicine Agency for hereditary metabolic diseases in the MetabERN network
Jean-Michel Heard, Charlotte Vrinten, Michael Schlander, Cinzia Maria Bellettato, Corine van Lingen, Maurizio Scarpa & the MetabERN collaboration group. Orphanet J Rare Dis 15, 3 (2020) doi:10.1186/s13023-019-1280-5
It was not the scope of this study to examine the impact of treatment costs, which was debated elsewhere, but rather to bring some indications on benefits for patient populations that are relevant to the assessment of treatment value. More accurate assessment of patient’s population benefit would require measuring markers of disease natural history and patient’s quality of life in treated patient cohorts and analysis of the results by all stakeholders, including HCPs, patients and families. Such methodological approach is needed to ensuring appropriate assessment of marketed treatment value and adapted decision on reimbursement. It is also desirable that end-users and public policy makers are involved at early steps of product development, in order to estimate the potential and/or expected value of the candidate treatments that are selected for development and future marketing. This might reduce the risk of developing and marketing products that do not adequately meet patient’s needs, and might optimize priority investments for OMPs.
It was not the scope of this study to examine the impact of treatment costs, which was debated elsewhere, but rather to bring some indications on benefits for patient populations that are relevant to the assessment of treatment value. More accurate assessment of patient’s population benefit would require measuring markers of disease natural history and patient’s quality of life in treated patient cohorts and analysis of the results by all stakeholders, including HCPs, patients and families. Such methodological approach is needed to ensuring appropriate assessment of marketed treatment value and adapted decision on reimbursement. It is also desirable that end-users and public policy makers are involved at early steps of product development, in order to estimate the potential and/or expected value of the candidate treatments that are selected for development and future marketing. This might reduce the risk of developing and marketing products that do not adequately meet patient’s needs, and might optimize priority investments for OMPs.
Sunday, January 26, 2020
Correlation of Visual Quality of Life With Clinical and Visual Status in Friedreich Ataxia
Afsharian P, Nolan-Kenney R, Lynch AE, Balcer LJ, Lynch DR.; J Neuroophthalmol. 2020 Jan 17. doi: 10.1097/WNO.0000000000000878.
Scores for the NEI-VFQ-25 were lower in patients with FRDA (n = 99) compared with published disease-free controls, particularly reduced in a subgroup of FRDA patients with features of early onset, older age, and abnormal visual function.
Scores for the NEI-VFQ-25 were lower in patients with FRDA (n = 99) compared with published disease-free controls, particularly reduced in a subgroup of FRDA patients with features of early onset, older age, and abnormal visual function.
Saturday, January 25, 2020
Modeling cardiac dysfunction of Friedreich's ataxia using ventricular sheets, tissues and chambers engineered from human pluripotent stem cells
Andy O.-T. Wong, Deborah K. Lieu, Gabriel K. Wong, Bimal Gurung, Wan Wai Tse, Kevin D. Costa, Camie W. Chan, Joseph D. Nabhan, Ronald A. Li; Journal of Pharmacological and Toxicological Methods, Volume 99, 2019, 106595, doi:10.1016/j.vascn.2019.05.175.
We conclude that these humanbased FRDA models provide a biomimetic platform suitable to facilitate the studies of disease pathogenesis and pharmaceutical testing.
We conclude that these humanbased FRDA models provide a biomimetic platform suitable to facilitate the studies of disease pathogenesis and pharmaceutical testing.
Friday, January 24, 2020
Frataxin deficiency induces lipid accumulation and affects thermogenesis in brown adipose tissue
Riccardo Turchi, Flavia Tortolici, Giulio Guidobaldi, Federico Iacovelli, Mattia Falconi, Stefano Rufini, Raffaella Faraonio, Viviana Casagrande, Massimo Federici, Lorenzo De Angelis, Simone Carotti, Maria Francesconi, Maria Zingariello, Sergio Morini, Roberta Bernardini, Maurizio Mattei, Piergiorgio La Rosa, Fiorella Piemonte, Daniele Lettieri-Barbato & Katia Aquilano; Death Dis 11, 51 (2020). doi:10.1038/s41419-020-2253-2
FXN deficiency in mice leads to clinical-pathological features parallel to those observed in diabetic patients. Among the metabolic parameters we have evidenced that the lipolytic and thermogenic activities of BAT are reduced, thus providing the possibilities of targeting BAT that might result in therapeutic benefits in FRDA.
FXN deficiency in mice leads to clinical-pathological features parallel to those observed in diabetic patients. Among the metabolic parameters we have evidenced that the lipolytic and thermogenic activities of BAT are reduced, thus providing the possibilities of targeting BAT that might result in therapeutic benefits in FRDA.
Thursday, January 23, 2020
Frataxin Structure and Function
Ignacio Hugo Castro, María Florencia Pignataro, Karl Ellioth Sewell, Lucía Daniela Espeche, María Georgina Herrera, Martín Ezequiel Noguera, Liliana Dain, Alejandro Daniel Nadra, Martín Aran, Clara Smal, Mariana Gallo, Javier Santos(2019). Frataxin Structure and Function. In: Harris J., Marles-Wright J. (eds) Macromolecular Protein Complexes II: Structure and Function. Subcellular Biochemistry, vol 93. Springer, Cham. DOI:10.1007/978-3-030-28151-9_13
By combining multiple experimental tools including high resolution techniques like NMR and X-ray, but also SAXS, crosslinking and mass-spectrometry, it was possible to build a reliable model of the structure of the desulfurase supercomplex NFS1/ACP-ISD11/ISCU/frataxin. In this chapter, we explore these issues showing how the scientific view concerning frataxin structure-function relationships has evolved over the last years.
By combining multiple experimental tools including high resolution techniques like NMR and X-ray, but also SAXS, crosslinking and mass-spectrometry, it was possible to build a reliable model of the structure of the desulfurase supercomplex NFS1/ACP-ISD11/ISCU/frataxin. In this chapter, we explore these issues showing how the scientific view concerning frataxin structure-function relationships has evolved over the last years.
Wednesday, January 22, 2020
Expresión de IRS2 en ratones afectados con ataxia de Friedreich
Gil Carceller, B. (2019). Grau en Biotecnologia, Universitat Politècnica de València. Escola Tècnica Superior d'Enginyeria Agronòmica i del Medi Natural.
CONCLUSIONES
1. Se ha realizado un estudio de perfil de expresión génica en tejidos aislados (cerebro, cerebelo, músculo, hígado y páncreas) de ratones wt versus ratones YG8R (ataxia de Friedreich) con el
objetivo de establecer una posible relación de la FRDA con la diabetes tipo 2.
2. Se ha establecido un gen constitutivo o Housekeeping, Hprt, que permite el análisis de la expresión génica en todos los tejidos en este estudio.
3. Se han observado diferencias significativas en cerebro (aumento de expresión de Irs1, Irs2, InsR e Igf1), cerebelo (disminución de expresión de Irs1, Irs2 e InsR) y músculo (disminución de
expresión de Irs1 y aumento de expresión de Irs2).
4. Se ha analizado Ucp2 por su implicación en la función mitocondrial y posible relación con la diabetes y la FRDA.
5. Existen evidencias que indican una posible relación de FRDA con la diabetes tipo 2 a nivel de expresión génica.
CONCLUSIONES
1. Se ha realizado un estudio de perfil de expresión génica en tejidos aislados (cerebro, cerebelo, músculo, hígado y páncreas) de ratones wt versus ratones YG8R (ataxia de Friedreich) con el
objetivo de establecer una posible relación de la FRDA con la diabetes tipo 2.
2. Se ha establecido un gen constitutivo o Housekeeping, Hprt, que permite el análisis de la expresión génica en todos los tejidos en este estudio.
3. Se han observado diferencias significativas en cerebro (aumento de expresión de Irs1, Irs2, InsR e Igf1), cerebelo (disminución de expresión de Irs1, Irs2 e InsR) y músculo (disminución de
expresión de Irs1 y aumento de expresión de Irs2).
4. Se ha analizado Ucp2 por su implicación en la función mitocondrial y posible relación con la diabetes y la FRDA.
5. Existen evidencias que indican una posible relación de FRDA con la diabetes tipo 2 a nivel de expresión génica.
Tuesday, January 21, 2020
Can this San Diego startup mimic exercise and fasting with a pill? Epirium raises $85M to find out
San Diego Union Tribune, BRITTANY MEILING, JAN. 13, 2020
A biotech startup in La Jolla has raised $85 million from notable science investors to investigate its idea for treating age-related diseases: a pill that mimics the effects of exercise and fasting.
The company, called Epirium Bio, says its scientists have discovered a new human hormone, which, when influenced, can trigger the production of more mitochondria. The depletion of the body’s mitochondria — a.k.a. “engines” of cells — is at the core of many age-related human illnesses.
To start, Epirium will be tackling rare muscle disorders, such as Duchenne muscular dystrophy, Becker muscular dystrophy and Friedreich’s ataxia.
A biotech startup in La Jolla has raised $85 million from notable science investors to investigate its idea for treating age-related diseases: a pill that mimics the effects of exercise and fasting.
The company, called Epirium Bio, says its scientists have discovered a new human hormone, which, when influenced, can trigger the production of more mitochondria. The depletion of the body’s mitochondria — a.k.a. “engines” of cells — is at the core of many age-related human illnesses.
To start, Epirium will be tackling rare muscle disorders, such as Duchenne muscular dystrophy, Becker muscular dystrophy and Friedreich’s ataxia.
Monday, January 20, 2020
Education and information needs for physicians about rare diseases in Spain
Enrique Ramalle-Gómara, Elena Domínguez-Garrido, María Gómez-Eguílaz, María Eugenia Marzo-Sola, José Luis Ramón-Trapero & Josefa Gil-de-Gómez; J Rare Dis 15, 18 (2020) doi:10.1186/s13023-019-1285-0
In conclusion, the study supports other investigations that have shown that clinicians lack easy access to educational opportunities and information resources regarding rare diseases. It is imperative that the public health system includes ongoing training on rare diseases in programs to improve the training of physicians in both primary care and specialized care.
In conclusion, the study supports other investigations that have shown that clinicians lack easy access to educational opportunities and information resources regarding rare diseases. It is imperative that the public health system includes ongoing training on rare diseases in programs to improve the training of physicians in both primary care and specialized care.
Sunday, January 19, 2020
Very-late-onset Friedreich’s ataxia: diagnosis in a kindred with late-onset cerebellar ataxia
Conor Fearon, Roisin Lonergan, Damien Ferguson, Susan Byrne, David Bradley, Yvonne Langan, Janice Redmond; Neurology 2020;20:55-58. doi:10.1136/practneurol-2019-002368
We present the clinical, imaging and genetic findings of a kindred with very-late-onset Friedreich’s ataxia and discuss the pitfalls and risk of misdiagnosis.
We present the clinical, imaging and genetic findings of a kindred with very-late-onset Friedreich’s ataxia and discuss the pitfalls and risk of misdiagnosis.
Saturday, January 18, 2020
Frataxin deficiency in Friedreich’s ataxia is associated with reduced levels of HAX-1, a regulator of cardiomyocyte death and survival
Francesca Tiano, Francesca Amati, Fabio Cherubini, Elena Morini, Chiara Vancheri, Sara Maletta, Silvia Fortuni, Dario Serio, Andrea Quatrana, Riccardo Luffarelli, Monica Benini, Giulia Alfedi, Luca Panarello, Alessandra Rufini, Nicola Toschi, Marina Frontali, Silvia Romano, Christian Marcotulli, Carlo Casali, Silvia Gioiosa, Caterina Mariotti, Alessia Mongelli, Mario Fichera, Ivano Condò, Giuseppe Novelli, Roberto Testi, Florence Malisan; Human Molecular Genetics, , ddz306, doi:10.1093/hmg/ddz306
Our results suggest that HAX-1 could be considered as a potential biomarker of cardiac disease in FRDA and the evaluation of its expression might provide insights into its pathogenesis as well as improving risk stratification strategies.
Our results suggest that HAX-1 could be considered as a potential biomarker of cardiac disease in FRDA and the evaluation of its expression might provide insights into its pathogenesis as well as improving risk stratification strategies.
Friday, January 17, 2020
PTC Therapeutics (PTCT) Presents At 38th Annual J.P. Morgan Healthcare Conference - Slideshow
seekingalpha. Jan. 16, 2020
Slide deck was published by PTC Therapeutics, Inc. in conjunction with this event.
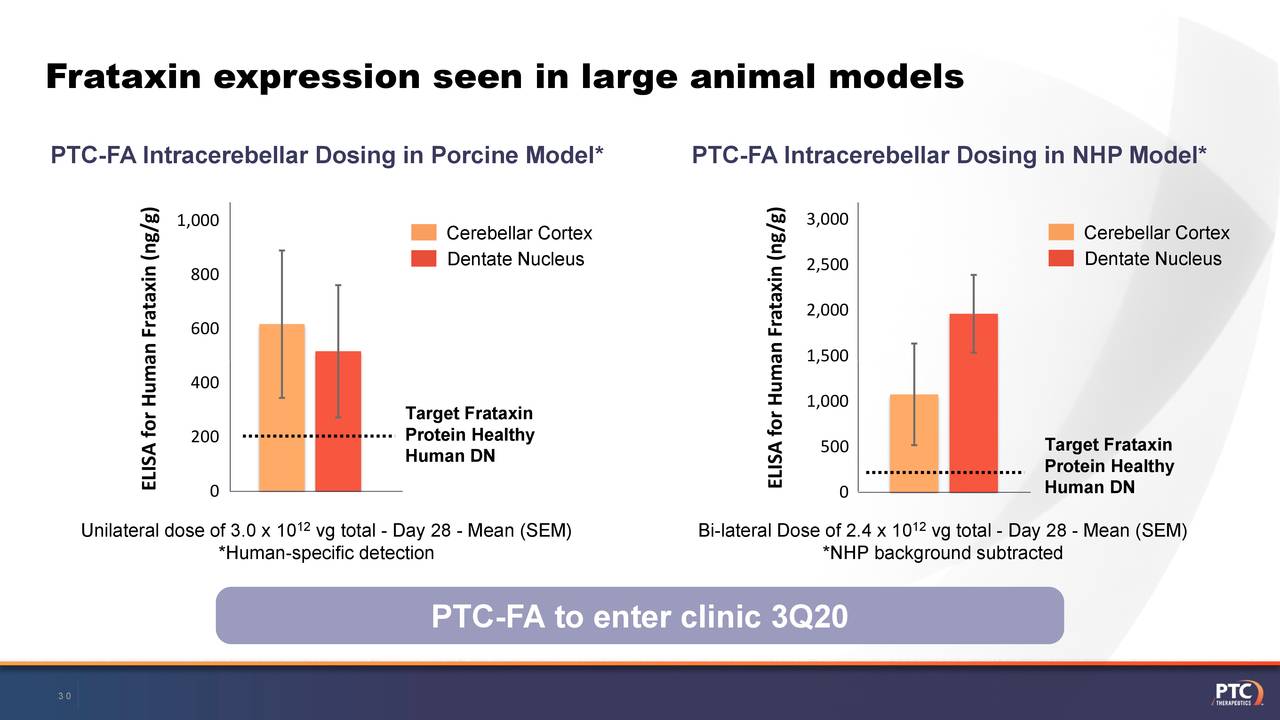
Slide deck was published by PTC Therapeutics, Inc. in conjunction with this event.

Thursday, January 16, 2020
Novartis' David Lennon on next steps for AveXis, selling Zolgensma's price to the public
BioPharma Dive (WEB 01/16/2020)
SAN FRANCISCO — Novartis sparked a new round of drug pricing criticism last May when the Swiss pharma revealed its new gene therapy Zolgensma would cost $2.1 million per patient.
Pipeline products to prioritize: "Those programs existed as part of the acquisition of AveXis. Since then, we started partnering with [the Novartis Institutes of Biomedical Research] to apply this platform to different diseases. The first one we announced is a project for Friedreich's ataxia, a muscle-wasting disease."
SAN FRANCISCO — Novartis sparked a new round of drug pricing criticism last May when the Swiss pharma revealed its new gene therapy Zolgensma would cost $2.1 million per patient.
Pipeline products to prioritize: "Those programs existed as part of the acquisition of AveXis. Since then, we started partnering with [the Novartis Institutes of Biomedical Research] to apply this platform to different diseases. The first one we announced is a project for Friedreich's ataxia, a muscle-wasting disease."
Safety and Efficacy of Interferon γ in Friedreich's Ataxia
Marinela Vavla MD PhD Maria Grazia D'Angelo MD PhD Filippo Arrigoni MD Nicola Toschi PhD Denis Peruzzo PhD Sandra Gandossini MD Annamaria Russo MD Eleonora Diella Stefania Tirelli PhD Roberto Salati MD Paolo Scarpazza MD Riccardo Luffarelli PhD Silvia Fortuni PhD Alessandra Rufini PhD Ivano Condò PhD Roberto Testi MD Andrea Martinuzzi MD PhD; Mov Disord. 2020 doi:10.1002/mds.27979
A relevant indication that emerges from the study, the previously studies, and from the numerous FRDA patients using IFNγ off label is the possible presence of nonresponders. A randomized withdrawal design is therefore best suited to increase the power of future clinical trials to definitively assess the efficacy of IFNγ treatment in FRDA
A relevant indication that emerges from the study, the previously studies, and from the numerous FRDA patients using IFNγ off label is the possible presence of nonresponders. A randomized withdrawal design is therefore best suited to increase the power of future clinical trials to definitively assess the efficacy of IFNγ treatment in FRDA
Wednesday, January 15, 2020
Predictors of loss of ambulation in Friedreich's ataxia
Christian Rummey, Jennifer M. Farmer, David R. Lynch; EClinicalMedicine, Volume 18, 2020, 100213, doi:10.1016/j.eclinm.2019.11.006.
We propose a stratification paradigm for time to LoA in FRDA. Concurrently, each step in a sequence of events represents a surrogate measure for future LoA. This will facilitate patient selection and stratification in clinical trials, and potentially enable study of LoA as a direct clinical outcome.
We propose a stratification paradigm for time to LoA in FRDA. Concurrently, each step in a sequence of events represents a surrogate measure for future LoA. This will facilitate patient selection and stratification in clinical trials, and potentially enable study of LoA as a direct clinical outcome.
Tuesday, January 14, 2020
PTC Therapeutics Provides Corporate Update and Highlights Pipeline Progress at 2020 J.P. Morgan Healthcare Conference
SOUTH PLAINFIELD, N.J., Jan. 13, 2020 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) today is providing a corporate update that will be presented at the 38th Annual J.P. Morgan Healthcare Conference on Wednesday, January 15th at 8:30 a.m.
PTC-FA gene therapy for Friedreich ataxia is progressing and is anticipated to enter the clinic in 3Q 2020.
In order to control and accelerate its gene therapy platform, PTC secured a 15-year lease on ~185,000 sq. ft. of space, which includes a state-of-the-art biologics production facility with supporting research and operations buildings in NJ. PTC expects manufacturing to begin at this facility in 2020.
PTC-FA gene therapy for Friedreich ataxia is progressing and is anticipated to enter the clinic in 3Q 2020.
In order to control and accelerate its gene therapy platform, PTC secured a 15-year lease on ~185,000 sq. ft. of space, which includes a state-of-the-art biologics production facility with supporting research and operations buildings in NJ. PTC expects manufacturing to begin at this facility in 2020.
MDA Awards Venture Philanthropy Funding of More Than $1M to AavantiBio to Develop Gene-Targeting Therapy for Friedreich's Ataxia
NEW YORK and GAINESVILLE, Fla., Jan. 13, 2020 /PRNewswire/ -- The Muscular Dystrophy Association (MDA) and AavantiBio, a biotechnology company developing a gene-targeting therapy for Friedreich's ataxia (FA), today announced the award of MDA Venture Philanthropy (MVP) funding totaling $1,076,232 to advance AavantiBio's phase 2 clinical trial of a gene-replacement therapy for the disease.
MDA's investment will help accelerate AavantiBio's mission and begin production of the clinical gene vector for its therapy program. Clinical trials are expected to begin in 2020.
MDA's investment will help accelerate AavantiBio's mission and begin production of the clinical gene vector for its therapy program. Clinical trials are expected to begin in 2020.
Monday, January 13, 2020
Mammalian iron–sulfur cluster biogenesis: Recent insights into the roles of frataxin, acyl carrier protein and ATPase-mediated transfer to recipient proteins
Nunziata Maio, Anshika Jain, Tracey A. Rouault; Current Opinion in Chemical Biology, Volume 55, 2020, Pages 34-44, doi:10.1016/j.cbpa.2019.11.014.
Highlights:
Iron–sulfur (Fe–S) clusters (ISCs) are metal cofactors involved in multiple, essential cellular processes.
Defined pathways assemble ISCs de novo in mammalian mitochondria and cytosol.
The acyl carrier protein is a component of the core ISC machinery.
Frataxin is an allosteric regulator that accelerates sulfur transfer from NFS1 to ISCU.
A chaperone/cochaperone system facilitates cluster transfer downstream of ISCU.
Insights into the role of FXN in FeeS biogenesis have arisen from recent structural and biochemical studies, an important future direction will be to search for drugs that can mimic FXN activity to treat patients with Friedreich’s ataxia, a disease caused by low FXN expression. The source of iron for Fe-S biogenesis remains unsettled, and future work will likely focus on how iron is delivered to the Fe-S biogenesis machinery. Much progress has been made, but many basic questions remain unanswered in the complex process of mammalian Fe-S biogenesis
Highlights:
Iron–sulfur (Fe–S) clusters (ISCs) are metal cofactors involved in multiple, essential cellular processes.
Defined pathways assemble ISCs de novo in mammalian mitochondria and cytosol.
The acyl carrier protein is a component of the core ISC machinery.
Frataxin is an allosteric regulator that accelerates sulfur transfer from NFS1 to ISCU.
A chaperone/cochaperone system facilitates cluster transfer downstream of ISCU.
Insights into the role of FXN in FeeS biogenesis have arisen from recent structural and biochemical studies, an important future direction will be to search for drugs that can mimic FXN activity to treat patients with Friedreich’s ataxia, a disease caused by low FXN expression. The source of iron for Fe-S biogenesis remains unsettled, and future work will likely focus on how iron is delivered to the Fe-S biogenesis machinery. Much progress has been made, but many basic questions remain unanswered in the complex process of mammalian Fe-S biogenesis
Friday, January 10, 2020
Retrotope Expands its Drug Pipeline with the First Dosing of RT001 in patients with Friedreich’s ataxia (FA)
LOS ALTOS, Calif., Jan. 09, 2020 (GLOBE NEWSWIRE) -- Retrotope announced today that it has dosed its first patient in a Phase 2/3 clinical trial of RT001 in Friedreich’s ataxia, the most common of the inherited ataxias. RT001, a stabilized fatty acid drug, has been shown to reduce lipid peroxidation leading to cell death in patients across a wide swath of degenerative diseases, including FA.
“This Phase 2/3 clinical trial is an important milestone in the development pathway of RT001” commented Peter G Milner, MD of Retrotope. “We believe the CPET is a very sensitive probe of the mitochondrial function and stamina of patients with this disease who suffer from profound fatigue due to mitochondrial neuropathy, myopathy and cardiomyopathy. FDA has agreed with us that CPET may be a primary endpoint in a marketing approval study, and will be evaluated for approval with other secondary, supportive efficacy measures and validated scales important to patient function.”
“This Phase 2/3 clinical trial is an important milestone in the development pathway of RT001” commented Peter G Milner, MD of Retrotope. “We believe the CPET is a very sensitive probe of the mitochondrial function and stamina of patients with this disease who suffer from profound fatigue due to mitochondrial neuropathy, myopathy and cardiomyopathy. FDA has agreed with us that CPET may be a primary endpoint in a marketing approval study, and will be evaluated for approval with other secondary, supportive efficacy measures and validated scales important to patient function.”
Thursday, January 9, 2020
Minoryx Therapeutics receives US FDA fast-track designation for leriglitazone in the treatment of X-ALD
Minoryx Therapeutics. Press Releases. Mataró, Barcelona, Spain and Charleroi, Belgium, January 9, 2020 – Minoryx Therapeutics
Leriglitazone (MIN-102), a novel, brain penetrant, orally bioavailable and selective PPARγ agonist, is currently in late-stage development for treatment of severe orphan CNS disorders, including X-ALD and Friedreich’s Ataxia. It previously received Orphan Drug Designation from EMA and FDA for both conditions
Leriglitazone (MIN-102), a novel, brain penetrant, orally bioavailable and selective PPARγ agonist, is currently in late-stage development for treatment of severe orphan CNS disorders, including X-ALD and Friedreich’s Ataxia. It previously received Orphan Drug Designation from EMA and FDA for both conditions
Large-scale contractions of Friedreich’s ataxia GAA repeats in yeast occur during DNA replication due to their triplex-forming ability
Alexandra N. Khristich, Jillian F. Armenia, Robert M. Matera, Anna A. Kolchinski, and Sergei M. Mirkin; PNAS first published January 7, 2020 Doi:10.1073/pnas.1913416117
Expansions of GAA repeats cause a severe hereditary neurodegenerative disease, Friedreich’s ataxia. In this study, we characterized the mechanisms of GAA repeat contractions in a yeast experimental system. These mechanisms might, in the long run, aid development of a therapy for this currently incurable disease. We show that GAA repeats contract during DNA replication, which can explain the high level of somatic instability of this repeat in patient tissues. We also provided evidence that a triple-stranded DNA structure is at the heart of GAA repeat instability. This discovery highlights the role of triplex DNA in genome instability and human disease.
Expansions of GAA repeats cause a severe hereditary neurodegenerative disease, Friedreich’s ataxia. In this study, we characterized the mechanisms of GAA repeat contractions in a yeast experimental system. These mechanisms might, in the long run, aid development of a therapy for this currently incurable disease. We show that GAA repeats contract during DNA replication, which can explain the high level of somatic instability of this repeat in patient tissues. We also provided evidence that a triple-stranded DNA structure is at the heart of GAA repeat instability. This discovery highlights the role of triplex DNA in genome instability and human disease.
Wednesday, January 8, 2020
Multiple mechanisms underpin cerebral and cerebellar white matter deficits in Friedreich ataxia: The IMAGE‐FRDA study
Louisa P. Selvadurai Louise A. Corben Martin B. Delatycki Elsdon Storey Gary F. Egan Nellie Georgiou‐Karistianis Ian H. Harding. Hum Brain Mapp. 2020; 1– 14. doi:10.1002/hbm.24921
For the first time, we examined the relative sensitivity and relationship between multiple white matter indices in Friedreich ataxia to more richly characterize disease expression and infer possible mechanisms underlying the observed white matter abnormalities.
Multiple mechanisms likely underpin white matter abnormalities in Friedreich ataxia, with differential impacts in cerebellar and cerebral pathways.
For the first time, we examined the relative sensitivity and relationship between multiple white matter indices in Friedreich ataxia to more richly characterize disease expression and infer possible mechanisms underlying the observed white matter abnormalities.
Multiple mechanisms likely underpin white matter abnormalities in Friedreich ataxia, with differential impacts in cerebellar and cerebral pathways.
Tuesday, January 7, 2020
Heart-In-A-Jar Platform Humanizing Preclinical Research
Bio-IT World.By Deborah Borfitz, January 6, 2020.
The pumping capability was one of the more compelling features of the three-dimensional (3-D) organ construct for AstraZeneca, which is looking to screen drug candidates for treating heart failure with preserved ejection fraction—a common condition especially among the elderly and in women—with no effective therapy available, says Li. Novoheart’s cardiac tissue engineering technology, known as the MyHeart Platform, also held appeal for Pfizer, which has used it to model both electrical and mechanical defects of the heart in patients with the neurodegenerative disease Friedreich’s ataxia (FRDA).
In a study published earlier this year in Stem Cell Research & Therapy, the FRDA models demonstrated utility in evaluating novel therapeutics and disease progression. Study results highlighted the potential of small molecules or gene therapy for suppressing or reversing the cardiac symptoms of FRDA.
The pumping capability was one of the more compelling features of the three-dimensional (3-D) organ construct for AstraZeneca, which is looking to screen drug candidates for treating heart failure with preserved ejection fraction—a common condition especially among the elderly and in women—with no effective therapy available, says Li. Novoheart’s cardiac tissue engineering technology, known as the MyHeart Platform, also held appeal for Pfizer, which has used it to model both electrical and mechanical defects of the heart in patients with the neurodegenerative disease Friedreich’s ataxia (FRDA).
In a study published earlier this year in Stem Cell Research & Therapy, the FRDA models demonstrated utility in evaluating novel therapeutics and disease progression. Study results highlighted the potential of small molecules or gene therapy for suppressing or reversing the cardiac symptoms of FRDA.
Monday, January 6, 2020
Researchers suggest a pathway to reverse the genetic defect of Friedreich's ataxia
Alexandra N. Khristich el al., "Large-scale contractions of Friedreich's ataxia GAA repeats in yeast occur during DNA replication due to their triplex-forming ability," PNAS (2019). Doi:10.1073/pnas.1913416117
"While these results were uncovered in a yeast model, they do provide us with a clue into the mechanism of DNA repeat instability in Friedreich's ataxia," said Alexandra Khristich, graduate student in Mirkin's lab and first author of the study. "I hope that our discovery would become a starting point for the potential development of therapeutic strategies that tip the balance toward DNA repeat contraction in patient tissues"
"While these results were uncovered in a yeast model, they do provide us with a clue into the mechanism of DNA repeat instability in Friedreich's ataxia," said Alexandra Khristich, graduate student in Mirkin's lab and first author of the study. "I hope that our discovery would become a starting point for the potential development of therapeutic strategies that tip the balance toward DNA repeat contraction in patient tissues"
Friday, January 3, 2020
Longitudinal Increases in Cerebral Brain Activation During Working Memory Performance in Friedreich Ataxia: 24-Month Data from IMAGE-FRDA
Rosita Shishegar, Ian H. Harding, Louise A. Corben, Martin B. Delatycki, Elsdon Storey, Gary F. Egan, Nellie Georgiou-Karistianis; Cerebellum (2020). doi:10.1007/s12311-019-01094-6
These findings provide the first evidence of increased longitudinal activation over time in the cerebral cortex in FRDA, compared with controls, despite comparable working memory performance. This finding represents a possible compensatory response in the ventral attention network to help sustain working memory performance in individuals with FRDA.
These findings provide the first evidence of increased longitudinal activation over time in the cerebral cortex in FRDA, compared with controls, despite comparable working memory performance. This finding represents a possible compensatory response in the ventral attention network to help sustain working memory performance in individuals with FRDA.
Subscribe to:
Comments (Atom)
